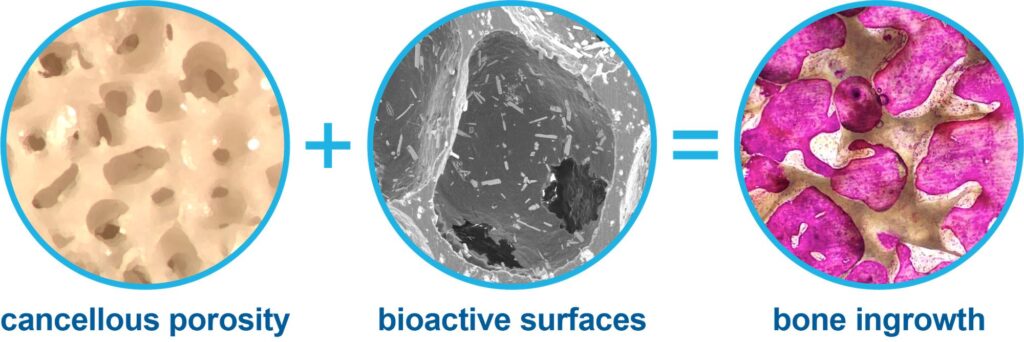
Grand Rapids, Mich., October 5th, 2022 — HAPPE Spine announces the addition of two more issued patents in a growing portfolio establishing HAPPE as the leader of next generation orthopaedic and spinal implants enabled by the HAPPE® (HydroxyApatite Porous PolyEtheretherketone) platform. The HAPPE material platform offers interconnected, cancellous porosity and bioactive surfaces with exposed hydroxyapatite to enable robust bone in-growth and on-growth confirmed in preclinical testing.
Dr. Ryan K. Roeder, Founder and CTO, said, “We are pleased the USPTO has granted utility patents for our implant design and manufacturing process that build upon previously issued and pending utility patents for our material composition. Together, these patents establish a strong intellectual property position for HAPPE in the emerging market for porous HA PEEK devices.”
U.S. Patent Number 11,179,243 protects implantable devices such as interbody spinal fusion cages that are constructed with (1) a thermoplastic polymer such as polyetheretherketone, (2) at least one region with interconnected porosity, and (3) bioactive particles such as hydroxyapatite integrated in the polymer and exposed on pore surfaces to encourage bone ingrowth.
U.S. Patent Number 11,426,904 protects a process for forming implants from thermoplastic polymers with regions of differing porosity. Unlike additive manufacturing of PEEK implants, the process enables molding of monolithic implants from powder-based raw materials such that there are no seams, joints or bonds in the implant, but rather a continuous polymer matrix between regions of relatively porous and dense material.
About HAPPE Spine:
HAPPE Spine LLC is a medical device development company that designs and commercializes innovative spinal implants utilizing the patented HydroxyApatite Porous PolyEtheretherketone (HAPPE®) material platform. HAPPE transforms polyetheretherketone from a hydrophobic, bioinert, and non-integrating material into a hydrophilic, bioactive, and osteointegrating material, confirmed by robust bone in-growth and on-growth in preclinical testing. The HAPPE INTEGRATE ™ interbody fusion system is the first interbody fusion cage to offer bone-like cancellous porosity with bioactive surfaces through the entire implant height to promote endplate-to-endplate osteointegration. INTEGRATE is designed to promote both load bearing and healing, as well as superior post-operative imaging. The HAPPE INTEGRATE interbody fusion system thus offers allograft-like qualities in a synthetic implant that can improve clinical outcomes in interbody spinal fusion by overcoming the problems of pseudoarthrosis, subsidence and imaging artifacts that limit current PEEK and titanium implants.
Learn more about HAPPE Spine and HAPPE® material platform at happeortho.com.
